What Is a Prospective Cohort Study? | Definition & Examples
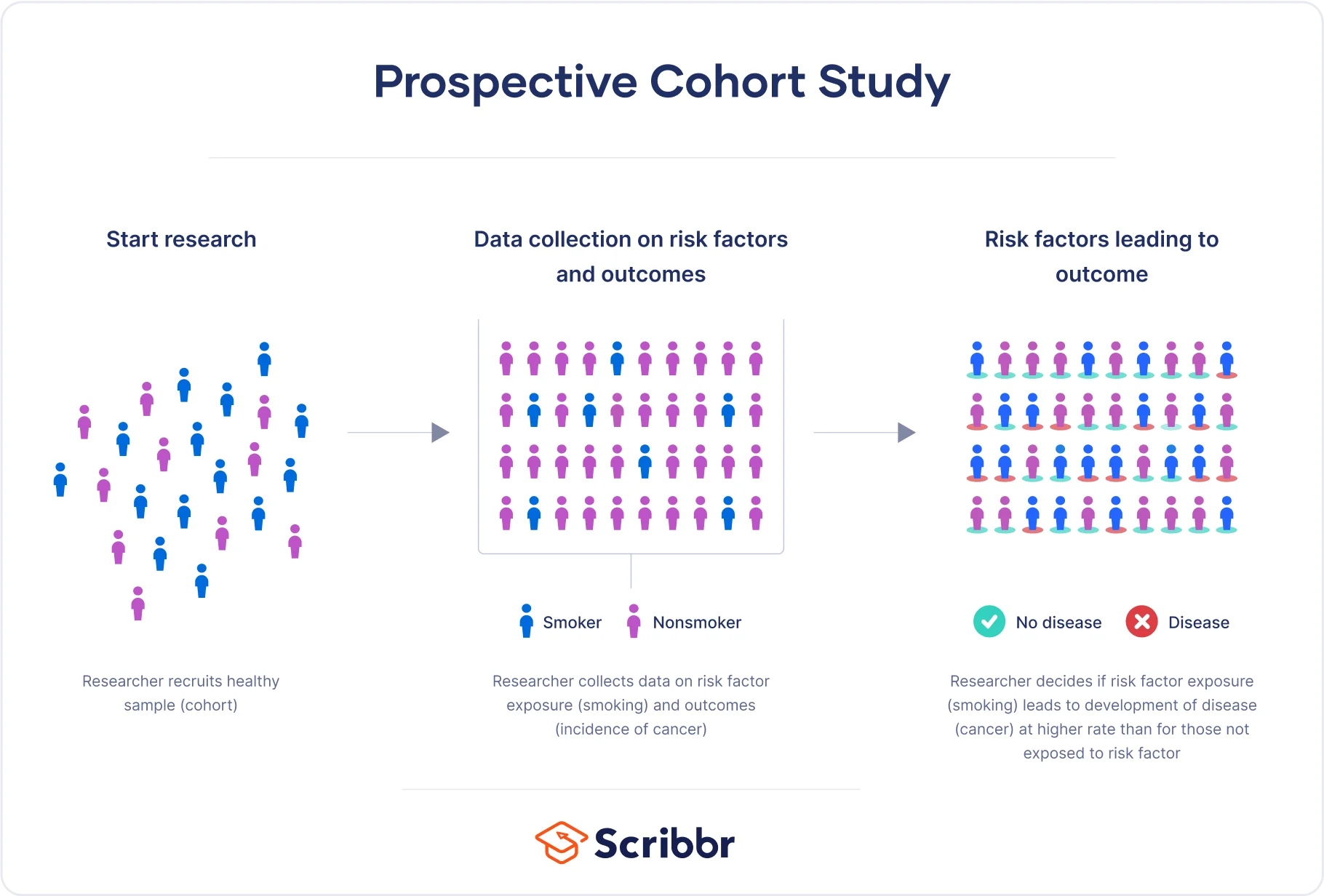
A prospective cohort study is a type of observational study focused on following a group of people (called a cohort) over a period of time, collecting data on their exposure to a factor of interest. Their outcomes are then tracked, in order to investigate the association between the exposure and the outcome.
Be careful not to confuse these with retrospective cohort studies, which look backwards in time.
It is crucial to note that in order to be considered a prospective cohort study, your participants must not possess the disease or health outcome being studied.
The study collected data on various risk factors, including diet, smoking, physical activity, and consumption of alcohol. It also tracked the occurrence of a variety of adverse health outcomes, such as heart disease and cancer.
The study provided many new and important insights into the relationship between lifestyle and health outcomes in women. It has led to many recommendations for policy around disease prevention and healthy habit promotion.
When to use a prospective cohort study
Prospective cohort studies are a type of observational study often used in fields related to health and medicine. While most observational studies are qualitative in nature, prospective cohort studies are often quantitative, as they use preexisting secondary research data. They can be used to conduct both exploratory research and explanatory research.
In prospective cohort studies, data is collected over time to compare the occurrence of the outcome of interest in those who were exposed to the risk factor and those who were not. This can help ascertain whether the risk factor could be associated with the outcome.
A prospective cohort study could be a good fit for your research if:
- You have access to a large pool of research subjects and are comfortable with a long timeline (i.e., many years).
- You are interested in the long-term effects of preventive or therapeutic treatment against exposure to a risk factor.
- Your research needs a stronger argument for potential causality than can be provided by retrospective cohort studies or case-control studies.
Cohort studies in general are more longitudinal in nature than case-control studies. Prospective cohort studies in particular often use secondary research data, such as existing medical records or databases, to initially identify a group of people. They then follow that group over a long period of time to see whether they are exposed to a particular risk factor of interest. Case-control studies rely on primary research, comparing a group of participants already possessing a condition of interest to a control group lacking that condition in real time.
Examples of prospective cohort studies
Prospective cohort studies are common in fields like medicine, epidemiology, and healthcare.
You recruit a group of healthy participants, all of whom were free of breast cancer at the beginning of your study. You then collect data on their level of physical activity and other lifestyle factors over a long period of time, tracking incidences of breast cancer. After many years, your results conclude that those who engaged in regular physical activity developed breast cancer at lower rates compared to those who were less active.
You recruit a group of healthy participants, all of whom were free of lung cancer at the beginning of your study. You then collect data on their exposure to air pollution, such as the levels of particulate matter or other pollutants in their local environment over a long period of time, tracking incidences of lung cancer. After many years, your results conclude that those who were exposed to higher levels of air pollutants had a higher risk of developing lung cancer compared to those who were not.
Advantages and disadvantages of prospective cohort studies
Prospective cohort studies can be a good fit for a wide range of research projects, particularly in health-related fields. However, they also have a few disadvantages to consider.
Advantages of prospective cohort studies
- Prospective cohort studies are better able to suggest causality than other types of observational studies due to their ability to establish temporality. Here, temporality allows researchers to determine that the exposure preceded the outcome, strengthening a claim for a cause-and-effect relationship between the two variables.
- While not immune, prospective cohort studies are less prone to research biases than other observational studies because they collect data before the outcome being studied occurs. This helps minimise the risk of biases like recall bias and selection bias, among others, and can even collect data on multiple outcomes over time, helping to identify new relationships between exposures and outcomes.
- Because prospective cohort studies usually rely on large groups of participants, they are more likely to use a sampling method to recruit a much more representative sample of the population. This leads to findings that are typically much more generalisable, with higher internal validity and external validity.
Disadvantages of prospective cohort studies
- Given their reliance on the recruitment of large groups of participants over long periods of time, prospective cohort studies can be both very time consuming and very expensive to conduct.
- While less prone to bias than other observational studies, they are not immune. In particular, prospective cohort studies are at risk for biases inherent to long-term studies like attrition bias and survivorship bias if participants drop out over time. The risk of confounding variables entering analysis over time can also affect the argument for causality, and measurement errors like omitted variable bias and information bias can further confound analysis.
- Like other experimental designs, prospective cohort studies can raise ethical considerations, particularly if the exposure of interest is harmful or if there is no known treatment for it. As a researcher, it is critical to ensure that participation in studies is voluntary, informed, and as safe as it can be for research subjects, and that they are able to give informed consent.
Frequently asked questions
Sources for this article
We strongly encourage students to use sources in their work. You can cite our article (APA Style) or take a deep dive into the articles below.
This Scribbr articleGeorge, T. (2023, June 19). What Is a Prospective Cohort Study? | Definition & Examples. Scribbr. Retrieved 9 April 2025, from https://www.scribbr.co.uk/research-methods/prospective-cohort-studies/
Colditz, G. A., & Hankinson, S. E. (2005). The Nurses’ Health Study: lifestyle and health among women. Nature Reviews Cancer, 5(5), 388–396. https://doi.org/10.1038/nrc1608